Key Takeaways:
- Genomic medicine is rapidly advancing with two key testing methods (rapid whole genome sequencing (rWGS) and biomarker testing) creating new challenges for state policymakers trying to keep up with the technology's potential for personalized healthcare.
- As of September, 17 state Medicaid programs now cover rWGS for critically ill infants. Four states expanded this to children under 21 in pediatric critical care units.
- Sixteen states mandate Medicaid coverage for biomarker testing. This mainly helps cancer patients with family history or risk factors, though coverage rules can be unclear.
- The One Big Beautiful Bill Act is making states hesitant to expand benefits due to costs. Meanwhile, lawmakers worry about genetic discrimination by insurers.
- The National Council of Insurance Legislators (NCOIL) is developing model legislation to prohibit life insurers from canceling policies based on genetic testing results and mandating applicants to submit to such tests, aiming for a consistent regulatory environment.
Advancements in gene sequencing have led to rapid progress in genomic medicine (a field of healthcare that utilizes the study of an individual's complete set of DNA, to understand and predict their health risks, diagnose diseases, and guide personalized treatment decisions), posing challenges for lawmakers and regulators to keep pace. Here’s an introduction to state policies concerning two essential genomic testing methods: rapid whole genome sequencing (rWGS) and biomarker testing.
Understanding Genomic Testing Technologies
Rapid Whole Genome Sequencing for Critical Care
rWGS comprehensively sequences an individual's entire genome to identify anomalies, mutations, or variations that explain illnesses, particularly in critically ill children. Its speed, delivering results in days or hours, is crucial for timely diagnosis and treatment, especially in NICUs and PICUs, where it can be life-saving.
Biomarker Testing Applications and Benefits
Biomarker testing is a targeted approach that identifies specific genetic variations, proteins, or molecules as indicators for disease presence, predisposition, or treatment response. Its applications are broad, including:
Early Detection and Prevention: Identifying genetic predispositions (e.g., BRCA1/2 for cancer).
Precision Medicine: Revolutionizing cancer treatment by targeting specific genetic alterations in tumors.
Monitoring Disease Progression: Tracking treatment effectiveness or chronic disease progression.
Pharmacogenomics: Optimizing medication choices and dosages based on an individual's genetic makeup. While rWGS offers a panoramic view for urgent diagnoses, biomarker testing provides focused insights for preventative strategies, personalized treatments, and improved long-term health outcomes.
State Medicaid Coverage for Genomic Testing
Rapid Whole Genome Sequencing (rWGS) Coverage Across State Programs
As of September 2025, 17 state Medicaid programs cover rWGS, primarily for critically ill children under one year old. Minnesota, North Carolina, Oklahoma, and Tennessee have expanded coverage to individuals under 21 who are admitted to or recently discharged from a pediatric critical care unit.
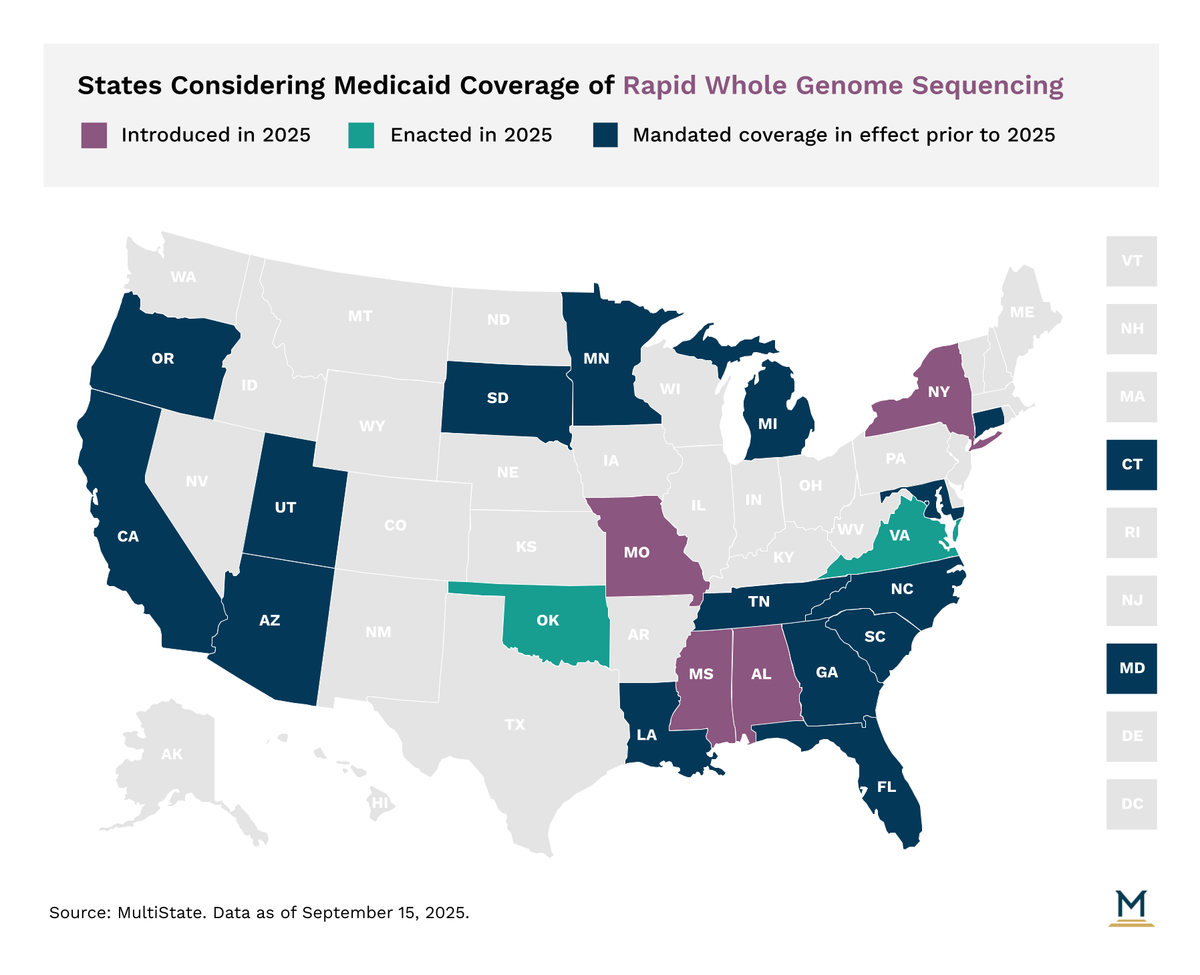
Biomarker Testing Medicaid Mandates
Sixteen states have mandated Medicaid coverage for biomarker testing. This coverage primarily extends to beneficiaries with a documented family history or risk factors for cancer, though some tests identify conditions beyond oncology, such as preeclampsia or Alzheimer's. The practical application of biomarker coverage can be ambiguous and is often subject to rigorous utilization management protocols like prior authorization and medical necessity reviews.
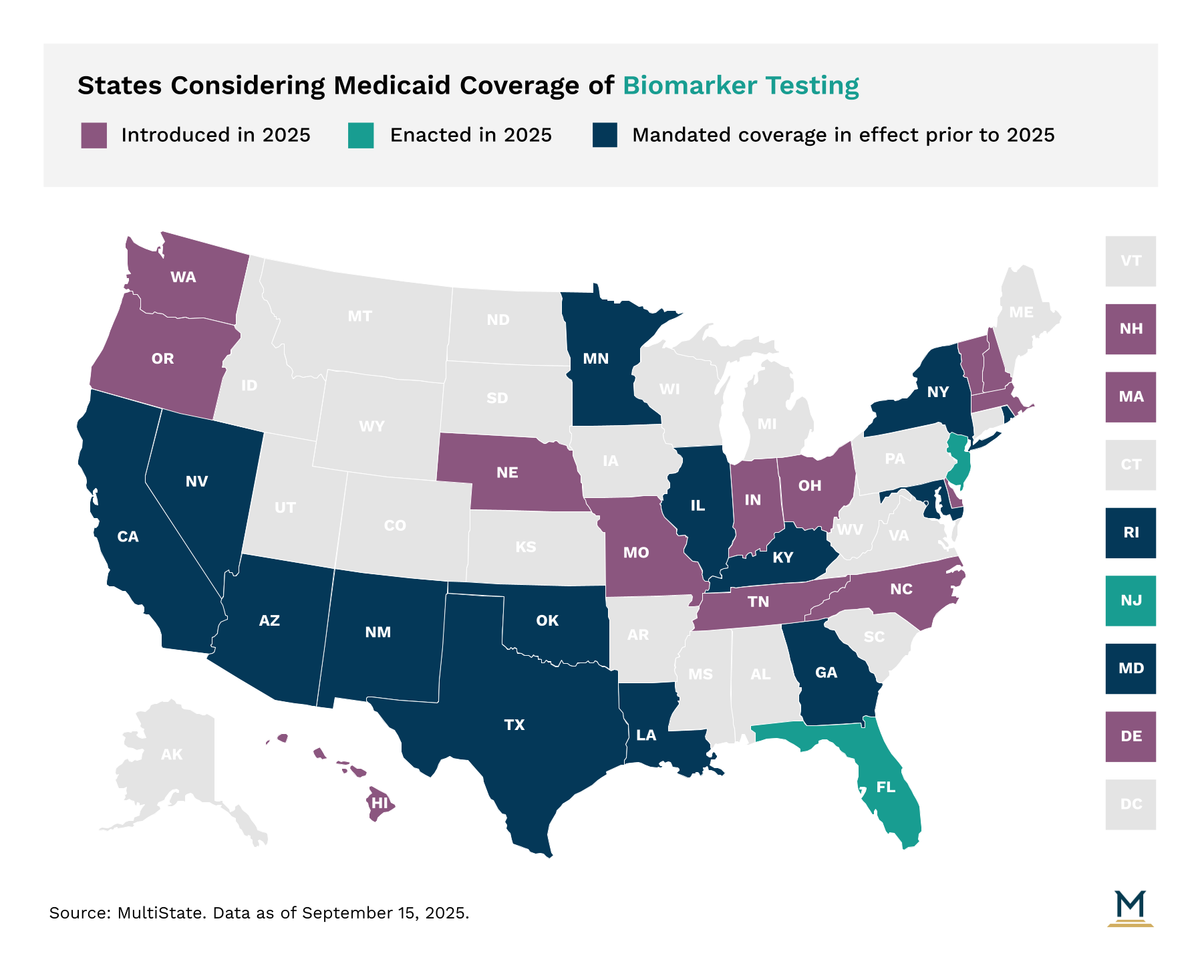
Future Legislative Developments and Insurance Protections
The One Big Beautiful Bill Act has introduced uncertainty for state Medicaid programs, making expanded benefits improbable due to potential fiscal ramifications. Legislators are increasingly focused on the implications of genetic data use by entities like life insurance companies. Maryland (House Bill 1007) and Illinois (Senate Bill 250) have enacted legislation prohibiting insurance carriers from discriminating based on genetic testing results for life, long-term care, and disability insurance. The National Council of Insurance Legislators (NCOIL) is developing model legislation to prohibit life insurers from canceling policies based on genetic testing results and mandating applicants to submit to such tests, aiming for a consistent regulatory environment.
Track Health Care Policy with StateVitals
StateVitals is the leading resource on how state governments are shaping healthcare policy and transforming care and delivery, brought to you by MultiState’s Health Care Policy Practice. MultiState’s policy and advocacy professionals are uniquely positioned to give you and your organization the big-picture view on state health policy reform, plus the latest trends on how policymakers are thinking about healthcare and similar emerging issues. Learn more about StateVitals, or schedule a demo here.






