
Health Care & Wellness
State Tobacco Legislation in 2025 Tackled Vaping, Nicotine Pouches, and More
February 5, 2026 | Geoff Hawkins

Key Takeaways:
In April, a federal district court dismissed a suit brought by pharmaceutical manufacturers against Minnesota Attorney General Keith Ellison and the state Board of Pharmacy. The suit challenged the constitutionality of Minnesota Statute §62J.96, which requires manufacturers to extend discount pricing under the 340B program to an unlimited number of contract pharmacies. In his ruling, the federal judge dismissed the suit, finding that manufacturers had a lack of standing in addition to Eleventh Amendment Immunity, which requires some connection between the officials and enforcement of challenged state law and of which the ruling finds that at this point in time neither the Attorney General or the Board of Pharmacy has enforced or threatened to enforce the statute.
As a reminder, the 340B Program requires pharmaceutical manufacturers to sell outpatient drugs at a discount to qualifying providers. By enabling these providers to pay lower prices for certain prescription drugs, the intent is to enable them to spend the savings realized from the 340B program to serve more patients and provide more comprehensive services.
Given the green light that Minnesota seemingly has for the time being to move forward with implementation of its own state law, a check-in on the state of play of 340B legislation moving within state legislatures is timely. While there were in excess of one hundred bills that touched the 340B program introduced this legislative session cycle, only 24 bills across 19 states have seen active movement. Of those bills, only 15 states saw or are seeing legislative action that would warrant a label of 340B reform.
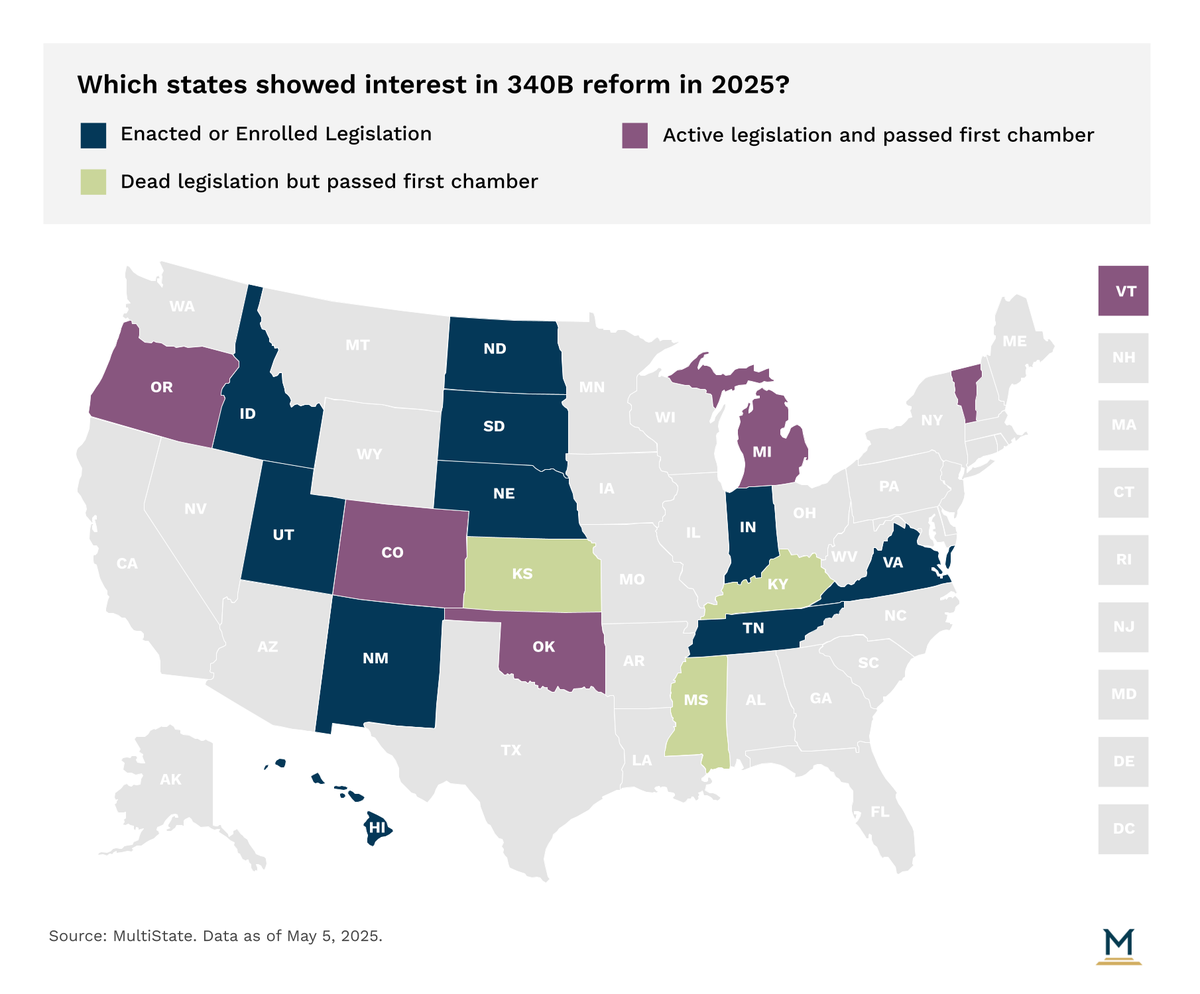
A few key themes have emerged in 2025 within these states that considered 340B legislation
Following a litany of judicial activity that opened the door for states to take the central role in providing oversight of how state regulated entities operate under the 340B program, states this year have continued to be proactive in prohibiting drug manufacturers, and in some cases wholesale distributors, from restricting or denying access to 340B-priced drugs for contract pharmacies of 340B covered entities. For background, pharmacies that contract with the 340B covered entity have historically received 340B pricing when acquiring drugs until pharmaceutical manufacturers have opted to challenge that notion in recent years. Nebraska, New Mexico, North Dakota, South Dakota, Tennessee and Utah have all already enacted legislation this session requiring manufacturers to extend 340B pricing to contract pharmacies. Hawaii recently engrossed their 340B reform effort that would establish similar requirements.
Notably, in Utah, Governor Spencer Cox (R) opted to neither sign nor veto SB 69 into law. Instead, allowing it to be enacted without his signature. In noting his decision to the Legislature, the Governor highlighted his opinion that the bill does not go far enough to ensure cost savings experienced by 340B covered entities are passed onto patients, nor is there enough transparency on how cost savings are being utilized by those entities. Importantly, Governor Cox maintains that these concerns outlined in the bill to be addressed should be addressed by Congress considering 340B is a federal program. This same logic has been leveraged by other Republican Governors in recent legislative sessions to allow but not endorse enactment of similar legislation.
As sessions look to the finish line in 2025, bills with similar language are engrossed in Colorado, Michigan, Oklahoma, and Vermont. Uniquely, in Michigan, SB 94 has a tie bar with SB 95, which is an unrelated bill specific to medical debt. However, in tying SB 94 to SB 95 when it was passed by the Senate, the House is eligible to only pass SB 95 if they also pass SB 94. Notably, SB 94, in addition to requirements for extending 340B pricing to contract pharmacies, also includes a reporting requirement for 340B covered entities to the Department of Health and Human Services on an annual basis that discloses any recent audits of their participation in the 340B program, certification of existing compliance with the 340B program, and a description of the 340B program’s impact in the covered entity’s community.
Transparency in the 340B program has become a focal point for lawmakers aiming to enhance accountability and better understand how savings from the program are used. Typically, this includes disclosing the aggregate acquisition cost of 340B drugs obtained, total payments received from insurers or patients, amounts paid to contract pharmacies, and how the resulting savings are deployed to benefit the local community. Such reporting requirements are often intended to ensure that 340B savings are being reinvested in patient care and community health initiatives, consistent with the program’s original intent.
In addition to Michigan’s SB 94 which includes a reporting requirement, Idaho has already enacted legislation this year with similar language. Notably, Idaho’s HB 136 will require any 340B covered entity to report six elements of data on an annual basis to the Department of Health and Welfare, the state’s controller office and the Attorney General. Those elements include: aggregate acquisition cost, aggregate payment amount received for drugs obtained, aggregate payment made to pharmacies under contract to dispense those drugs, the number of claims for prescription drugs dispensed under the 340B program, and how savings are utilized.
Similarly, Colorado (SB 71 | SB 124) has engrossed legislation moving through their Legislature. In Indiana, the General Assembly enrolled similar legislation. Although, efforts to enhance transparency in the 340B program in Indiana are further compounded by an executive order issued by Governor Mike Braun (R) in January, which calls for the state to investigate and determine rulemaking authority to ensure 340B hospitals meet 340B program eligibility requirements, prohibit duplicate discounts, and ensure adherence to Medicaid Exclusion File and Cost Report. The report will also examine how off-site, outpatient facilities are utilized as part of the program. If these efforts are successful, these states would join Minnesota, Maine and Washington that have some level of required reporting from 340B covered entities.
While those two themes remain dominant this year across states, other 340B topics continue to arise. For instance, Maryland’s legislation (SB 357 | HB 424) to expand the state’s Prescription Drug Affordability Review Board’s (PDAB) upper payment limit (UPL) setting authority requires the PDAB to consider impacts to 340B covered entities when considering UPLs. This theme has been similarly discussed in other states with UPL setting authority or with legislation introduced with a similar intent due to concern that a UPL would inadvertently reduce savings that a 340B covered entity would be able to pull from.
In another perspective, the most significant theme seen in past years, prohibiting a health plan, PBM or third-party payer from reimbursing a 340B entity for 340B drugs at a rate lower than that paid for the same drug to entities that are not 340B entities, has fallen a bit into the background. Only Oklahoma has engrossed legislation that would establish such a protection.
Without any expected intervention from Congress in the near future, expect 340B legislation to remain a core focal point for a multitude of stakeholders as we head into the final weeks of legislative sessions across the country. MultiState’s team is actively identifying and tracking health care issues so that businesses and organizations have the information they need to navigate and effectively engage. If your organization would like to further track health care or other related issues, please contact us.

February 5, 2026 | Geoff Hawkins

January 27, 2026 | Lisa Kimbrough

January 27, 2026 | Amber Thyson