
Health Care & Wellness
Alcohol Legislation Takes A New Turn: Canned Cocktails, Direct-to-Consumer Shipping, and More
February 12, 2026 | Kerrie Zabala
May 15, 2025 | Mary Kate Barnauskas

Key Takeaways:
In recent years, states have increasingly turned to Prescription Drug Affordability Boards (PDABs) as a policy tool to address prescription drugs costs. Eleven states have established a PDAB or similar drug cost control entity.
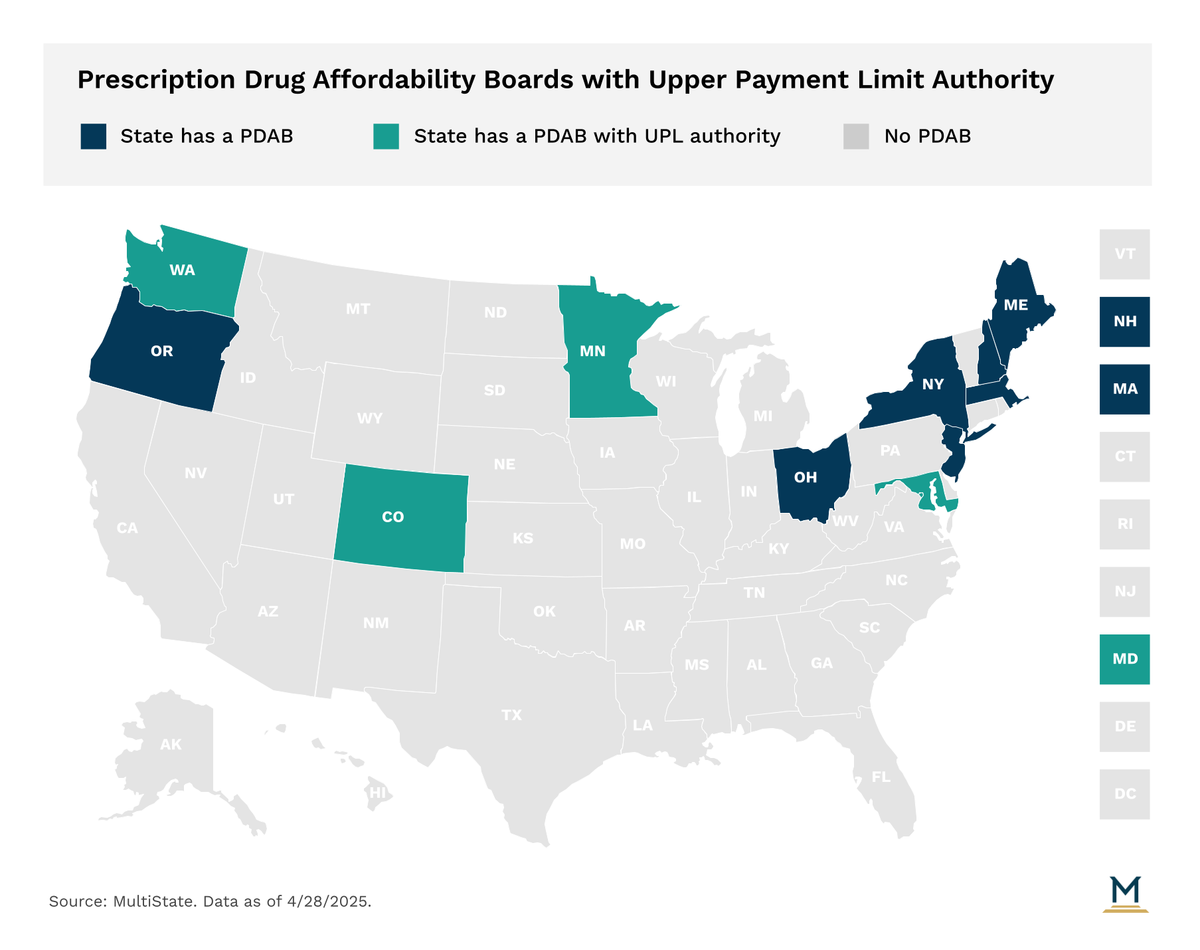
A Prescription Drug Affordability Board (PDAB) is a state entity charged with addressing prescription drug costs for states and consumers. PDABs are typically composed of appointed individuals with expertise in healthcare and the pharmaceutical supply chain. Several states have stakeholder advisory councils consisting of representatives from industries along the supply chain and consumers who the PDAB consults with. PDAB authority varies by state, and can include making policy recommendations, creating reports, setting spending benchmarks, conducting affordability/cost reviews, or setting upper payment limits (UPLs).
Colorado, Maryland, Minnesota, & Washington: Conduct affordability reviews of eligible drugs and set UPLs.
Oregon: Conducts affordability reviews of eligible drugs.
Maine & New Hampshire: Set spending targets for specific drugs and recommend policies to public payers to meet the targets.
New Jersey: Formulate legislative and regulatory policy recommendations to address the high costs of prescription drug products.
Massachusetts: If the Executive Office of Health and Human Services cannot negotiate supplemental rebate agreements with drug manufacturers, the manufacturer may be referred to the Health Policy Commission (HPC) to review the value of the drug and propose a supplemental rebate.
New York: Medicaid can refer drugs to the Drug Utilization Review Board (DURB) to negotiate supplemental rebates if spending on a drug that is expected to exceed the Medicaid drug cap or if a newly launched drug meets certain thresholds.
Ohio: The prescription drug transparency and affordability advisory council is no longer active after its statute was amended in 2021, but topics related to its previous work can be examined by the Joint Medicaid Oversight Committee (JMOC).
Four states, Colorado, Maryland, Minnesota, and Washington, have PDABs with UPL authority, though no state has implemented a UPL yet. PDABs with UPL authority are tasked with identifying high cost drugs that meet thresholds set in statute for affordability review. During affordability reviews, PDABs analyze drug pricing, spending trends, utilization data, and stakeholder input to determine if a drug is unaffordable. If a PDAB determines a drug is unaffordable, it may choose to set a UPL. UPLs cap the amount that purchasers and payers within a state can be required to pay for certain drugs. UPLs have been a central point of debate in the PDAB with questions around legal authority, enforcement, and market impact.
States have continued to debate PDABs in 2025 with new legislation and legal challenges shaping the future of such policy. In Virginia, the General Assembly passed legislation to establish a PDAB with UPL authority for the second year in a row, but was met with a second veto from Gov. Glenn Youngkin (R). In his veto message, Gov. Youngkin expressed concerns on the impact of UPLs on patient access and medical innovation.
The Maryland General Assembly has also considered UPLs this year as it passed legislation to expand the PDAB’s UPL authority. Currently, the Maryland PDAB only has authority to set UPLs for public entities, including Medicaid. In order to set such UPLs, the PDAB was required to draft a plan of action for setting UPLs for approval from the General Assembly’s Legislative Policy Committee. In October 2024, the PDAB received approval of their UPL Action Plan and has been moving towards conducting cost review studies for 6 selected drugs, which could lead to the state’s first UPLs. Meanwhile, in April 2025, the General Assembly passed HB 424/SB 357, which would allow the PDAB to set UPLs beyond public purchasers following successful implementation. The bill also adds additional reporting requirements on UPL impact and requires the PDAB to consider drug shortages, rare diseases, and 340B entities in setting UPLs. The legislation now awaits the signature of Governor Wes Moore (D), who is expected to sign it.
In a closely watched case, Colorado’s PDAB faced a challenge from a drug manufacturer after one of its drugs was deemed unaffordable and selected for a UPL. The manufacturer claimed that the PDAB was unconstitutional as it conflicted with federal law and violated due process. On March 28, 2025, a U.S. District Court dismissed a motion for summary judgment in the case. The court determined that the company lacked standing since the manufacturer is not directly regulated by the statute since UPLs only apply to downstream transactions, not the manufacturer’s point of sale. The manufacturer has filed an appeal, but Colorado has indicated that it will proceed with UPL rulemaking in the meantime.
This year has already been significant for PDABs and UPLs, and more action is expected as PDABs progress to setting UPLs. However, legal and implementation challenges remain, and industry and lawmakers will be watching closely to assess the impact of PDAB and UPL policy at the state level.
The ever-evolving state health policy landscape will continue to influence how health care organizations make business decisions. MultiState’s team pulls from decades of expertise to help you effectively navigate and engage. MultiState’s team understands the issues, knows the key players and organizations, and we harness that expertise to help our clients effectively navigate and engage on their policy priorities. We offer customized strategic solutions to help you develop and execute a proactive multistate agenda focused on your company’s goals. Learn more about our Health Care Policy Practice.

February 12, 2026 | Kerrie Zabala

February 5, 2026 | Geoff Hawkins

January 27, 2026 | Lisa Kimbrough